
B
rito
AE
et
al
.
1022
R
ev
A
ssoc
M
ed
B
ras
2017; 63(12):1019-1023
Treatment with six doses of 55 kBq/Kg of intravenous
Ra223 injections every 30 days is recommended for pa-
tients with mCRPC and bone metastases.
D
iscussion
and
perspectives
At the moment only five other medications, in addition
to Ra223, which produce a demonstrated increase in
survival in patients with mCRPC (docetaxel, cabazitaxel,
abiraterone, enzalutamide, sipuleucel-T) are available. In
view of this scenario, Ra223 stands out as a treatment
with few contraindications and acceptable adverse effects,
and an excellent option for mCRPC patients.
Although the studies presented here use a dose of 50
kBq/kg, the commercial dose was adjusted to 55 kBq/kg
to meet the standardization criteria.
13
(
D
)
Only one study carried out re-treatment with Ra223
in mCRPC patients.
14
(
B
) Although it is a possibility, since
the study showed safety, we do not recommend repeat-
ing the treatment until further studies are performed.
Studies are being conducted to validate the concomi-
tant use of Ra223 with other therapies. We highlight the
combination of Ra223 treatment with enzalutamide (phase
III studies), abiraterone (phase II: NCT02097303), deno-
sumab (phase II: NCT02366130), bicalutamide (phase II:
NCT02582749) and radiotherapy (phase II: NCT02484339).
In addition, studies in asymptomatic patients are being
performed (NCT03002220).
Ra223 is also being studied to treat other diseases such
as osteosarcoma (NCT01833520), multiple myeloma
(NCT02928029) and breast cancer (phase II: NCT02258451).
As soon as these studies are available, we will update
this guideline.
C
onflict
of
interest
The authors state that there is no conflict of interest re-
garding this review.
R
eferences
1. ANVISA. Registro ANVISA nº 170560104 – XOFIGO [cited 2017 Apr 9].
Available from:
https://www.smerp.com.br/anvisa/?ac=prodDetail&anvisaId=170560104.
2.
Nilsson S. Radium-223 therapy of bone metastases in prostate cancer. Semin
Nucl Med. 2016; 46(6):544-56.
3.
Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-
targeted radium-223 in symptomatic, hormone-refractory prostate cancer:
a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol.
2007; 8(7):587-94.
4. Nilsson S, Franz L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Two-year
survival follow-up of the randomized, double-blind, placebo-controlled phase
II study of radium-223 chloride in patients with castration-resistant prostate
cancer and bone metastases. Clin Genitourin Cancer. 2013; 11(1):20-6.
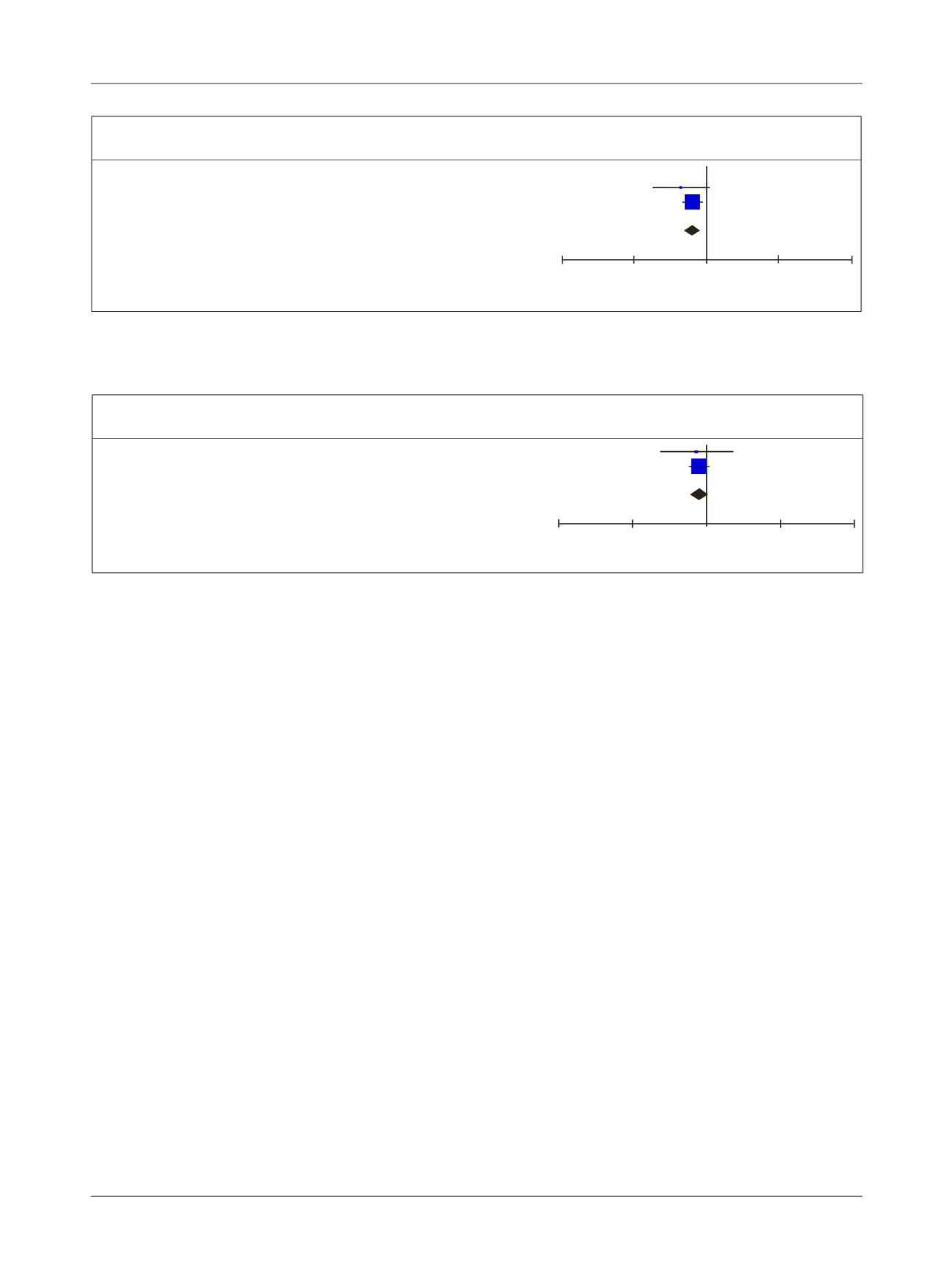
FIGURE 1
Summary of the studies comparing the use of Ra223 versus placebo in the treatment of mCRPC regarding the outcome of
increased survival.
FIGURE 2
Summary of the studies comparing the use of Ra223 versus placebo in the treatment of mCRPC regarding the outcome of number
of bone events.
Study or
subgroup
Radio 223
Placebo
Risk difference
Risk difference
Events Total
Events Total
Weight M-H, Fixed, 95CI
M-H, Fixed, 95CI
Nilsson 2007 17
33 18
31 7.2% -0.07 [-0.31, 0.18]
-1
-0.5
0
0.5
1
Favors [radio 223]
Favors [placebo]
Sartor 2014 200 614 116 307 92.8% -0.05 [-0.11, 0.02]
Total (95CI)
647
338 100.0% -0.05 [-0.11, 0.01]
Total events
219
134
Heterogeneity Chi
2
= 0.02, df = 1 (p=0.90); I
2
= 0%
Test for overall effect: Z = 1.55 (p=0.12)
Study or
subgroup
Radio 223
Placebo
Risk difference
Risk difference
Events Total
Events Total Weight M-H, Fixed, 95CI
M-H, Fixed, 95CI
Nilsson 2007 0
0
0
0
0.0% -0.74 [-0.90, 0.58]
-1
-0.5
0
0.5
1
Favors [radio 223]
Favors [placebo]
Nilsson 2013 23
33 27
31 7.2% -0.17 [-0.37, 0.02]
Parker 2013 333 614 195 307 92.8% -0.09 [-0.16, -0.03]
Total (95CI)
647
338 100.0% -0.10 [-0.16, -0.04]
Total events
356
222
Heterogeneity Chi
2
= 0.60, df = 1 (p=0.44); I
2
= 0%
Test for overall effect: Z = 3.05 (p=0.002)
















